Inorganic chemistry (Gary L. Miessler)
풀었던 부분만 올립니다
3.12
Select from each set the molecule or ion having the smallest bond angle, and briefly explain your choise:
a. NH3, PH3, or AsH3
b.
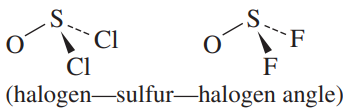
c. NO2- or O3
d. ClO3- or BrO3-
풀이:
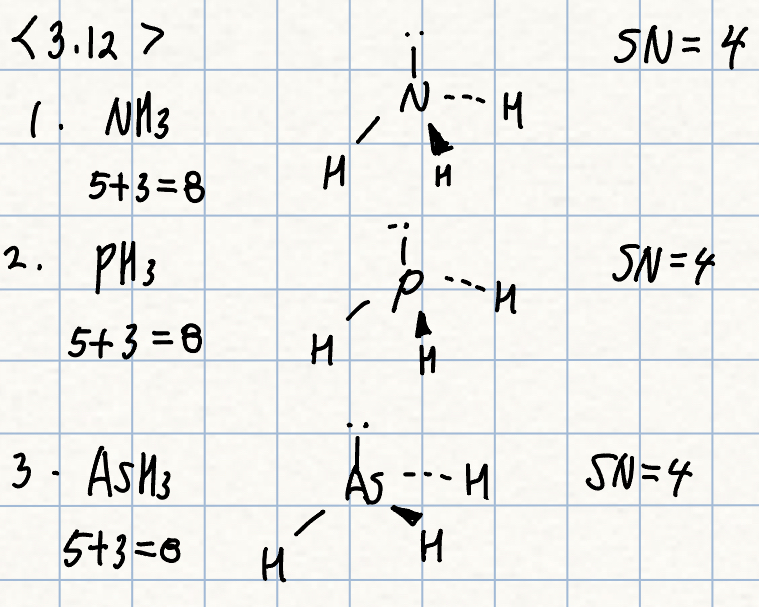
a. AsH3 should have the smallest angle, since it has the largest central atom.
- This minimizes the bond pair-bond pair repulsions and allows a smaller angle.
- Arsensic is also the least electronegative central atom, allowing the electrons to be drawn out farther and lowering the repulsions further.
- Acture angles: AsH3 = 91.8도, PH3 = 93.8도, NH3 = 106.6
b. Cl is larger than F, and F is more electronegative and should pull the electrons farther from the S
- So the F-S-F angle should be smaller in OSF2.
- This is consistent with the experimental data: the F-S-F angle in OSF2 is 92.3도,
and the Cl-S-Cl angle in OSCl2 is 96.2도
c.
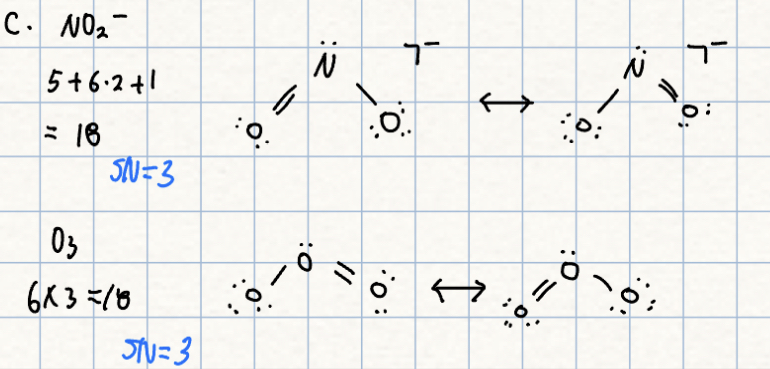
- NO2- has rather variable angles (!15도 and 132도) in different salts. The sodium salt(115.4도) has a slightly smaller angle than O3(116.8도). The N-O electronegativity difference should pull electrons away from N, reducing the bp-bp repulsion and the angle.
d.
- BrO3- (104도) has a slightly smaller angles than ClO3- (107도).
- since it has a larger central atom
- In addition, the greater electronegativity of Cl holds the electrons closer, and increases bp-bp repulsion.
3.20
Predict and sketch the structure of the (as yet) hypothetical ion IF3 2-



