(쪽수: 번역판 기준)
p. 16 Exercise 2.1
Determine the energy of the transition from nh = 3 to nl = 2 for the hydrogen atom, in both joules and cm-1
(a common unit in spectroscopy, often used as an energy unit, since v is proportional to E ).
This transition results in a red line in the visible emission spectrum of hydrogen .
(Solutions to the exercises are given in Appendix A .)
(* RH = Rydberg constant for hydrogen = 1.097 * 107 m-1 = 2.179 * 10-18 J = 13.61 eV)
풀이:
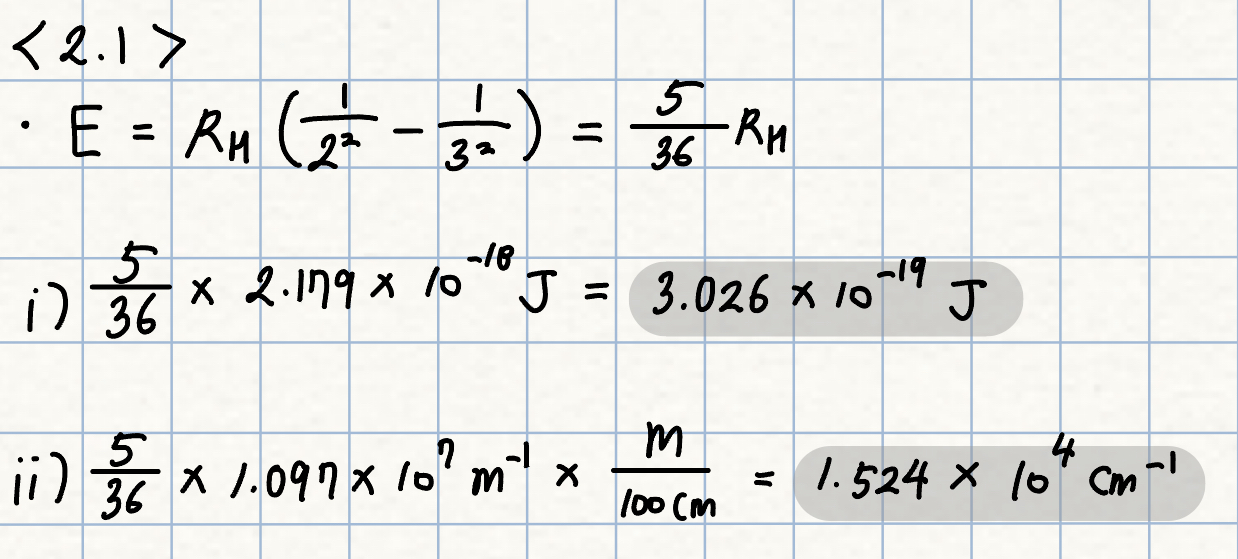
p. 29 Example 2.1
Nodal structure of pz The angular factor Y is given in Table 2.3 in terms of Cartesian coordinates:

Describe the angular nodal surfaces.
p. 29 Example 2.1
Nodal structure of d x2-y2

p. 29 Exercise 2.2
Describe the angular nodal surfaces for a dz2 orbital, whose angular wave function is
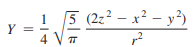
p. 29 Exercise 2.3
Describe the angular nodal surfaces for a d xz orbital, whose angular wave function is

풀이:

p.32 Example 2.2
1> Oxygen
With four p electrons, oxygen could have two unpaired electrons, or it could have no unpaired electrons.
a. Determine the number of electrons that could be exchanged in each case, and find the Coulombic and exchange energies.
b. Which state is lower in energy?
풀이:

p.33 Exercise 2.4
A third possible state for the p4 configuration would be

Determine the Coulombic and exchange energies of this state, and compare its energy with the energies of the states determined in the preceding example. Draw a sketch showing the relative energies of these three states for oxygen's p4 configuration.
풀이:

p. 33 Exercise 2.5
A nitrogen atom, with three 2p electrons, could have three unpaired electrons, or it could have one unpaired electron.
a. Determine the number of electrons that could be exchanged in each case and the Coulombic and exchange energies. Which state would be lower in energy?
b. A third possible state for a 2p3 configuration would be

Determine its Coulombic and exchange energies, and compare the energy of this state with the energies determined in part a.
풀이:

p. 37 Example 2.3
(1) Oxygen
Use Slater's rules to calculate the shielding constant and effective nuclear charge of a 2p electron.
(2) Nickel
Use slater's rules to calculate the shielding constant and effective nuclear charge of a 3d and 4s electron.
(원자번호: 28)
풀이:

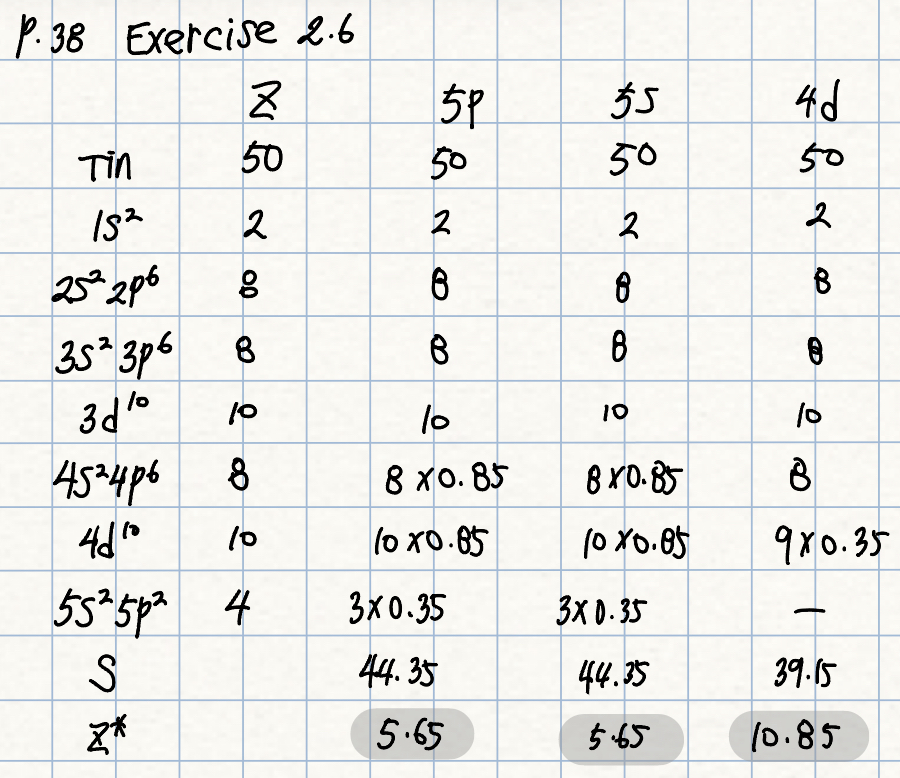
p. 38 Exercise 2.6
Calculate the effective nuclear charge on a 5s, 5p and 4d electron in a tin atom
(원자번호: 50)
풀이:
p. 38 Exercise 2.7
Calculate the effective nuclear charge on a 7s, 5f, and 6d electron in a uranium atom
(원자 번호: 92)
풀이:

p. 40
Rich's approach에 대하여 설명하라.
- the difference in E between 1e and 2e in the same orbital (Πc), first e (ms = -1/2) & second e (ms = +1/2) for 4s
- 3d : shorter most probable distances from the nucleus than 4s
- Most probable distance from the nucleus ↑ ∝ n ↑
- Z* ↑ ∝ E stabilized
- E of 3d more stabilized than 4s
→ (n-1)d & ns
- Formation of a positive ion → reducing shielding (Z* ↑)
- E: (n-1)d & ns
- Remaining es at d orbital
- Always removed first for e in the highest n (4s)

p. 43 Exercise 2.8
Explain why all three graphs in Fig 2.14 have maxima at 4 electrons and minima at 5 electrons.
풀이:




